Abstract
Introduction: Concizumab is a humanized, recombinant monoclonal antibody that acts to inhibit tissue factor pathway inhibitor to enhance coagulation. Patients with hemophilia A or B with inhibitors (HAwI/HBwI) received concizumab as a once-daily subcutaneous prophylactic treatment in the phase 3 explorer7 study (NCT04083781). In the explorer7 primary analysis, concizumab prophylaxis was shown to reduce the annualized bleeding rate for patients with either HAwI or HBwI when compared with no prophylaxis. Concizumab was also considered to be safe and well tolerated for HAwI/HBwI, with the latter patient population having the greatest unmet medical need. Subcutaneous administration of concizumab using a pen device may contribute to a lower treatment burden when compared with intravenous factor replacement therapy. Here, we present data on patient-reported treatment burden and patient preference from patients with HAwI/HBwI treated with concizumab in explorer7.
Methods: A total of 133 patients aged ≥12 years were either randomized 2:1 to concizumab prophylaxis (arm 2; n=33) or no prophylaxis (arm 1; n=19) or assigned to non-randomized concizumab prophylaxis treatment (arms 3 or 4; n=81) based on the treatment they were receiving before entering the study. Patients were prompted to complete the Hemophilia Treatment Experience Measure (Hemo-TEM; Brod et al. abstract presented at ISTH 2022 [PB0654]) questionnaire at baseline (Week 0) and Week 24. In addition, patients were asked to complete the hemophilia patient preference questionnaire (H-PPQ) at Week 24. Results from both questionnaires were reported as exploratory endpoints in the study. Due to technical reasons, a number of patient-reported outcomes records could not be collected for statistical analysis. Analysis of covariance was applied to estimate mean change in Hemo-TEM domain and total scores from baseline to Week 24 in arms 1 and 2, with treatment, type of haemophilia and bleeding frequency prior to screening as factors, and the baseline value as a covariate. Descriptive statistics were used to describe the results of the H-PPQ questionnaire.
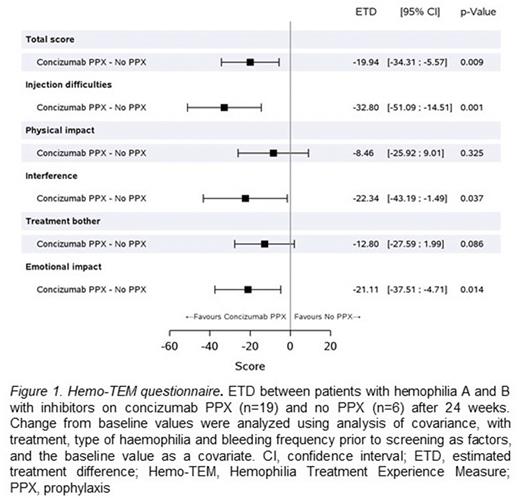
Results: The Hemo-TEM total score improved in patients on concizumab prophylaxis (n=19) from baseline to Week 24 (mean estimate -17.0 points [95% CI: -23.7; -10.3]), while patients on no prophylaxis (n=6) did not experience an improvement (mean estimate 3.0 points [95% CI: -9.4; 15.3]). The estimated treatment difference in Hemo-TEM total score between the two arms was statistically significant (-19.9 points [95% CI: -34.3; -5.6]; P=0.009; Figure 1). Improvements were observed across all Hemo-TEM domains for patients on concizumab prophylaxis, with significant improvements in the domains "injection difficulties", "interference", and "emotional impact" (Figure 1). A total of 99 patients received the H-PPQ questionnaire; 77 (78%) indicated that they preferred concizumab over their previous treatment, while only one patient (1%) preferred their previous treatment (16 patients did not respond, and 5 patients had no preference). Out of the 83 patients who responded, 93% preferred concizumab over their previous treatment. The three main reasons for their treatment preference were "fewer bleeds" (75%), "require less time" (43%), and "less painful to inject" (33%).
Conclusion: A lower treatment burden was observed for daily concizumab prophylaxis as patients generally reported better Hemo-TEM domain and total scores. For the Hemo-TEM total score, the estimated treatment difference between concizumab and no-prophylaxis arms was statistically significant. Most patients who responded to the H-PPQ questionnaire preferred concizumab prophylaxis over their previous treatment. Intravenous injection of replacement factor products poses a major burden to hemophilia patients. The observed improvements in treatment burden with subcutaneous administration of concizumab may have the potential to also improve treatment adherence and thereby achieve better clinical outcomes for patients with hemophilia. The combination of effective prophylaxis and reduced bleeding (especially in HBwI) together with lower treatment burden of subcutaneous delivery resulted in a clear patient preference in favor of concizumab.
Disclosures
Hampton:Novo Nordisk: Honoraria; CSL: Honoraria; Bayer: Honoraria. Knoebl:Alexion: Honoraria; Novo Nordisk: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Research Funding; CSL Behring: Honoraria; Shire/Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ablynx/Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Biotest: Honoraria. Odgaard-Jensen:Novo Nordisk: Current Employment. Stasyshyn:Shire/Takeda: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria; Octapharma: Consultancy; Sanofi: Honoraria, Research Funding; CSL-Behring: Research Funding; Novo Nordisk: Consultancy, Honoraria, Research Funding. Thaung Zaw:Novo Nordisk: Current Employment. Neergaard:Novo Nordisk: Current Employment. Zulfikar:Novo Nordisk: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; Roche: Consultancy; Sobi: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Research Funding, Speakers Bureau; Genveon: Honoraria; Octapharma: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal